The pharmaceutical industry relies on patients to take daily pills, but this manual process fails half the time because human memory is unreliable. This design flaw creates a massive efficiency gap where patients perform unpaid work to manage their health, costing billions in preventable emergencies. Instead of creating apps to nag patients, companies must switch to autonomous implants and injectables that deliver medicine automatically without user effort. This shift guarantees the medicine works and removes the burden of adherence entirely.
A First Principles Deconstruction of Medical Compliance
Target Audience: Pharma Executives, Digital Health Strategists, Product Architects, Clinical Operations Leads.
Part I: The Deconstruction (The Socratic Scalpel)
Goal: Dismantle the industry’s “Stuck Belief” that non-compliance is a behavioral flaw rather than a design defect.
The “Bad Patient” Myth
The pharmaceutical industry operates on a fundamental category error: the belief that non-compliance is a behavioral defect rather than a structural failure. When a system fails 50% of the time—as chronic medication adherence does after six months, according to the World Health Organization—it is not a user error; it is a design flaw. The industry has spent decades trying to “fix the patient” through education, gamification, and behavioral nudges, failing to recognize that the patient is unreliable “human middleware” in a precise chemical delivery loop.
We currently rely on biological agents (patients) to execute precise pharmacokinetic schedules, a task for which the human brain is evolutionarily ill-suited. In any other safety-critical industry—aviation, nuclear power, or high-frequency trading—relying on manual human intervention for daily maintenance would be considered negligence. Yet in medicine, we label the failure of this manual process as “non-compliance,” shifting the burden of the system’s design failure onto the end-user. We don’t need better patients; we need to fire the patient from the job of drug delivery.
The Five Whys of Failure (RFPA Step 1)
To understand why the “Adherence Crisis” persists despite billions in investment, we must apply the Robust First Principles Analyst (RFPA) protocol, starting with the Five Whys. This diagnostic chain reveals that the problem is not biological or psychological, but economic.
Why is compliance low? Because the therapeutic loop requires manual human actuation every 24 hours.
Why is the loop manual? Because the Oral Solid Dose (OSD)—the pill—is the dominant standard for medication delivery.
Why is the pill dominant? Because it is exceptionally cheap to manufacture and stable to ship.
Why do we prioritize manufacturing cost over delivery reliability? Because the business model is built on “Volume of Units Sold” rather than “certainty of therapeutic outcome.”
The Root Cause: We are optimizing for Factory Efficiency (the cost to make the pill), not Therapeutic Efficacy (the probability the molecule reaches the receptor).
This analysis reveals that “Adherence” is a problem created by the solution itself. We are currently stuck in Path A (Optimization), trying to make “pill swallowing” easier. The First Principles imperative is Path B (Deletion): question the existence of the pill. If the delivery mechanism (the pill) requires a level of consistency that the user (the human) cannot provide, the mechanism must be deleted.
The “Nagging” Economy
The “Nagging Economy”—the estimated $50 billion sector comprising adherence apps, smart pill bottles, glow-caps, and nurse call centers—is the industrialization of “Sustaining Innovation.” These tools attempt to patch a fundamentally broken user interface (the daily dose) rather than eliminating the friction entirely. In the vocabulary of the Musk Loop, this is “paving the cow path”—automating and optimizing a process that should not exist.
Consider the “Smart Pill Bottle.” It uses sensors and Bluetooth to track when a patient opens the cap, sending data to a cloud dashboard. While technologically impressive, it is functionally absurd. It adds cost ($50+ per unit), complexity (batteries, syncing, data privacy), and cognitive load (notifications) to a process that is already failing due to friction. It is a Band-Aid Innovation that reinforces the legacy dependence on the daily oral dose. True innovation does not make it easier to remember the pill; it makes the pill unnecessary. We must stop building better alarm clocks and start building autonomous delivery systems.
Part II: The ID10T Audit (Efficiency Gap Analysis)
Goal: Quantify the economic and physiological cost of the current “Daily Dose” model using the ID10T Index.
Calculating the “Compliance Tax”
The “Compliance Tax” is the rigorous quantification of the systemic waste generated by the current reliance on manual patient adherence. It is not an abstract concept; it is a direct financial levy on the healthcare system caused by the failure of the “Daily Dose” interface. According to the CDC and NIH, the direct cost of prescription non-adherence in the United States ranges from $100 billion to $300 billion annually. This figure represents the cost of avoidable hospitalizations, emergency room visits, and escalated disease states resulting from patients failing to act as reliable delivery mechanisms.
In human terms, this design failure results in approximately 125,000 preventable deaths per year. This is equivalent to a fully loaded jumbo jet crashing every single day. If any other consumer product—a car, a toaster, a phone—had a user interface failure rate that killed 125,000 people annually, it would be recalled immediately. The fact that the “Daily Pill” remains the standard of care is a testament to the industry’s focus on Manufacturing Ease over User Reality.
The ID10T Index of the Oral Solid Dose
The ID10T Index (Inefficiency Delta in Operational Transformation) measures the gap between the Current Commercial Price of a process and its Theoretical Minimum Cost (physics limit). For medication adherence, the inefficiency is driven by “Shadow Labor”—the uncompensated work we force patients to perform.
The Numerator (Current Commercial Reality):
The true cost of the daily pill is not just the pharmacy price. It includes the cost of the “Compliance Infrastructure” required to prop up the failing system. This includes:
Nursing time spent on adherence counseling.
The cost of “Smart” packaging and reminder apps.
The catastrophic cost of “Rescue Care” (ER visits) when the system fails.
The Denominator (The Physics Limit):
The theoretical minimum cost of maintaining a therapeutic blood concentration is the cost of the molecule plus the energy required to deliver it, with zero human labor.
The Labor Floor (Shadow Labor): We assign a value to the patient’s time using the Standard L1 Manual Labor Rate ($25/hr).
Task: Remember, Locate, Open, Swallow, Log, Refill.
Time: Conservative estimate of 2 minutes per day.
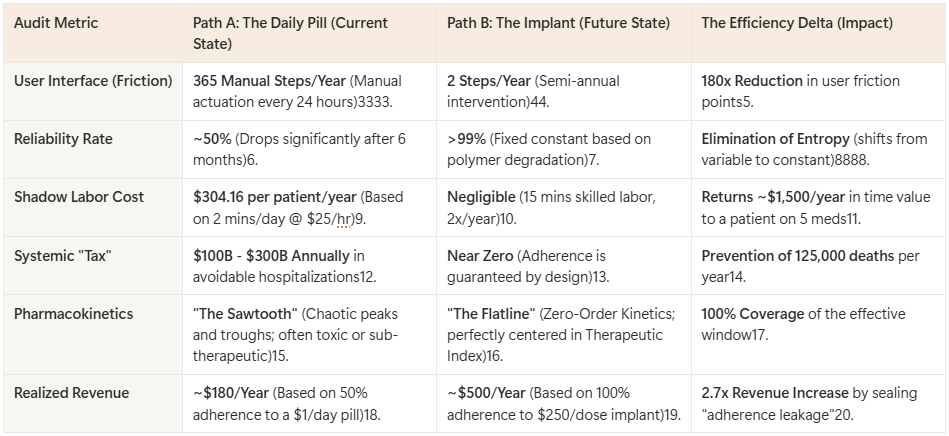
Calculation: $25/hr * (2/60 hours) * 365 days = $304.16 per patient per year.
The Bits Floor: The cost of digital monitoring (if autonomous) approaches $0.01.
The Efficiency Delta:
The current system essentially imposes a $304 annual labor tax on every patient for every chronic medication they take. If a patient is on 5 medications, they are performing $1,500 worth of uncompensated labor annually to act as a manual servo in the pharma supply chain. By switching to a Long-Acting Injectable (LAI) or Implant that requires intervention only twice a year (15 minutes of L2 Skilled labor), we reduce the failure points by a factor of 180x (365 events vs. 2 events).
The “Entropy of Adherence”
Adherence is ultimately a problem of thermodynamics: systems tend toward disorder (entropy) unless energy is applied. In the context of daily medication, “Entropy” is the statistical probability of missing a dose.
The Rule of Compounding Failure:
Every manual step in a process introduces a probability of failure. If a patient has a 90% reliability rate for a single task (high for a human), a daily regimen often involves three distinct micro-steps: (1) Remembering the time, (2) Locating the medication, (3) Physically ingesting it.
Reliability Calculation: $0.9 \times 0.9 \times 0.9 = 0.729$ (72.9% reliability per day).
Over a week, the probability of Perfect Adherence drops to near zero.
Conclusion:
You cannot “educate” a human to overcome the laws of thermodynamics. You cannot “gamify” your way out of entropy. The only way to increase the reliability of the system is to delete the steps. By moving from a daily oral dose (365 steps/year) to a semi-annual implant (2 steps/year), you structurally eliminate the opportunity for entropy to enter the system. We must stop trying to make the patient more disciplined and start making the therapy more autonomous.
Part III: The Path Choice (JTBD Elevation)
Goal: Pivot from “Getting Patients to Take Meds” (Path A) to “Ensuring Therapeutic Levels” (Path B).
Defining the Job-to-be-Done
The Jobs-to-be-Done (JTBD) framework demands we separate the Solution (the pill) from the Job (the biological outcome). The pharmaceutical industry has historically operated at Level 1 Abstraction, defining the job as “Help me remember to take my medication.” This low-level definition inevitably leads to low-level solutions: reminder apps, vibrating caps, and automated pill dispensers. These solutions fail because they assume the patient wants to perform the task of adherence. They do not.
To unlock disruptive innovation, we must elevate the job to Level 3: “Maintain therapeutic blood concentrations of MoleculeX within the effective window.”
This simple linguistic shift fundamentally alters the design constraints. If the job is to “maintain blood concentration,” the patient is no longer a necessary actor in the process; they are simply the vessel. The patient does not want to take medicine; they want to be medicated. The ideal user experience for a chronic therapeutic is invisibility. Every interaction required by the patient is evidence of a design failure in the delivery mechanism.
The Innovation Battlefield: Path A vs. Path B
The industry stands at a divergence point between two distinct innovation paths.
Path A: The “App Trap” (Sustaining Innovation)
This path accepts the “Daily Dose” as a fixed constraint and attempts to optimize the patient’s behavior around it.
The Strategy: “We need to engage the patient.”
The Tactics: Gamification, financial incentives for adherence, Bluetooth-enabled packaging, AI chatbots for “coaching.”
The Result: “Paving the Cow Path.” We are spending billions to make an inefficient, entropy-prone process slightly more tolerable. This is the “Faster Horse” approach to medicine.
Path B: “Zero-UI Therapeutics” (Disruptive Innovation)
This path rejects the daily dose and deletes the requirement for patient labor.
The Strategy: “We need to eliminate the patient.”
The Tactics: Long-Acting Injectables (LAIs), Subdermal Implants, Bio-erodible Microspheres, Gene Editing.
The Result: Structural Elimination of Non-Adherence. When a drug is onboarded via a 6-month implant, adherence becomes a constant (100%) rather than a variable. The “interface” disappears entirely.
The Market Reality Check
Before committing to Path B, we must apply the Market Reality Check to ensure we are not proposing science fiction.
The Existence Test:
Does “Zero-UI” technology currently exist in the market? Yes.
HIV Prevention: Apretude (Cabotegravir extended-release) replaces 365 daily pills with 6 bi-monthly injections.
Metabolic Health: Ozempic/Wegovy (Semaglutide) normalized the weekly injection over the daily pill for mass-market consumers.
Contraception: Nexplanon (Etonogestrel implant) provides 3 years of “Zero-UI” efficacy.
The Physics Test:
Is it physically possible to reformulate any drug into a long-acting format?
The Volume Constraint: The primary limitation is the physical volume of the active pharmaceutical ingredient (API) required. High-potency molecules (requiring micrograms/milligrams per day) are viable candidates for implants or depot injections. Low-potency “bulk” molecules (requiring grams per day, like Metformin) currently violate the volume constraints of a comfortable implant.
The Verdict: Path B is valid for High-Potency Chronic Therapies (Antipsychotics, ARVs, Statins, Hormones) but requires further materials science breakthroughs for high-volume compounds.
Part IV: The Reconstruction (First Principles Solutions)
Goal: Build the “Autonomous Therapeutic Stack.”
The Physics of “Set and Forget”
The reconstruction of the therapeutic model begins with a shift in pharmacokinetics from “Pulse Dosing” to “Linear Release.” The Oral Solid Dose (OSD) inherently produces a “Sawtooth” profile in blood plasma concentration. Every time a patient swallows a pill, drug levels spike (often causing toxicity or side effects) and then degrade exponentially (often dropping below the effective threshold) until the next dose. This requires the patient to act as a precise metronome to keep the drug within the Therapeutic Index (TI)—the narrow window between efficacy and toxicity.
Physics dictates that relying on a manual, pulsatile input to maintain a steady homeostatic state is an inefficient design. “Set and Forget” therapeutics utilize Zero-Order Kinetics, where the drug is released at a constant rate independent of concentration. This creates a “Flatline” profile, keeping the drug concentration perfectly centered within the Therapeutic Index 100% of the time. This not only deletes the need for adherence but often improves clinical outcomes by eliminating the “troughs” where viral replication or symptom breakthrough occurs.
The “Invisible Infrastructure”
To achieve Zero-Order Kinetics without human intervention, we must build an “Invisible Infrastructure” inside the body. This stack consists of three physical layers:
The Depot (The Storage Tank): This is the high-density reservoir of the Active Pharmaceutical Ingredient (API). Innovations in materials science, such as in-situ forming hydrogels and bio-erodible polymers (like PLGA), allow us to store months of medication in a volume smaller than a matchstick. The key constraint here is API Potency—the drug must be potent enough that a 6-month supply fits within a few milliliters of volume.
The Governor (The Rate Controller): This is the mechanism that enforces linear release. It replaces the patient’s memory with physics. In passive systems, this is achieved through diffusion-controlled membranes or erosion-based matrixes. In active systems (MEMS), a micropump acts as the governor, dispensing precise nanoliter volumes based on a pre-programmed schedule.
The Sentinel (The Feedback Loop - Future State): The ultimate evolution is the “Closed Loop” system. Here, a biosensor (The Sentinel) monitors a biomarker (e.g., glucose, viral load) and signals The Governor to adjust the dose in real-time. This mimics the function of a biological organ (like the pancreas), converting the therapeutic from a static “product” into a dynamic “service.”
Deleting the Process (Musk Loop Step 2)
Applying the Musk Loop (RFPA) to this new infrastructure reveals the massive efficiency gains achieved through deletion. By moving the “factory” inside the patient, we delete the entire legacy supply chain of adherence.
Deleted: The monthly trip to the pharmacy (and the associated breakage/churn risk).
Deleted: The “pill organizer” and the cognitive load of managing a daily schedule.
Deleted: The “Adherence Counselor” and the entire “Nagging Economy” of reminder apps.
Deleted: The anxiety of “Did I take my pill today?”
The result is a phase shift in the nature of compliance. It moves from a Variable (dependent on human will, memory, and chaotic life events) to a Constant (dependent only on the known degradation rate of a polymer). Reliability increases from ~50% (human limit) to >99% (physics limit).
Part V: The Execution (Real Options Strategy)
Goal: How to transition a Pharma portfolio from “Pills” to “Platforms.”
The “Option to Switch” (Portfolio Strategy)
Transitioning from a volume-based oral solids business to a value-based autonomous delivery business represents a massive Innovator’s Dilemma. To manage this risk, we employ Real Options Analysis (ROA). We must view the current portfolio of Oral Solid Doses (OSD) not as the future, but as the funding mechanism for the “Option to Switch.”
The Declining Asset: The “Daily Pill” is a depreciating asset class. As generic competition erodes margins and payers demand “outcome-based pricing,” the value of a pill that works 50% of the time (due to adherence failure) trends toward zero.
The Strategic Trigger: We establish a portfolio-wide “Half-Life Trigger.” For any molecule in the pipeline with a biological half-life of less than 24 hours (requiring daily dosing), we automatically purchase the “Option to Reformulate.” This means allocating a small, defined budget (e.g., $5M) to test feasibility for Long-Acting Injectable (LAI) or Implant delivery. This is not a commitment to launch, but a commitment to buy the right to launch a superior format if the market shifts.
The Cash Cow Strategy: Use the reliable cash flow from the “Nagging Economy” products (legacy pills) to purchase these innovation options. We are effectively shorting our own legacy products to go long on the disruption.
Overcoming the “Business Model Problem”
The most significant barrier to Path B is not technology, but the business model. The industry is addicted to the “Daily Active User” (DAU) metric of pills sold. Curing a patient or dosing them once every 6 months appears, on a spreadsheet, to destroy volume.
Reframing Volume vs. Value: We must shift the metric from “Units Shipped” to “Therapeutic Days Guaranteed.” A daily pill sold for $1.00/day generates $365/year if adhered to. In reality, with 50% adherence, it generates $180/year and results in treatment failure. A 6-month implant priced at $250/dose generates $500/year and guarantees 100% adherence. The “Volume” is lower (2 units vs 365 units), but the “Captured Value” is higher because the leakage of non-adherence is sealed.
Bio-SaaS (Biology as a Service): This enables a subscription model. The payer does not pay for the drug; they pay for “Metabolic Control” or “Viral Suppression.” If the implant fails, the pharma company bears the cost. If it works, the revenue is recurring and predictable. This aligns the incentives of the manufacturer (efficacy) with the payer (health outcome) and the patient (zero friction).
The Roadmap to Zero
The transition to Zero-UI Therapeutics follows a three-phase horizon:
Phase 1: The Reformulation (Years 1-2)
Target: High-potency, off-patent molecules with known adherence issues (Statins, Antipsychotics).
Action: Reformulate into 30-day extended-release injectables.
Goal: Capture the “Adherence Premium” in established markets.
Phase 2: The Implant (Years 3-5)
Target: High-value chronic therapies (HIV, Contraception, Metabolic).
Action: Launch 6-month to 1-year bio-erodible implants.
Goal: Establish “Set and Forget” as the standard of care, making daily pills look archaic and negligent.
Phase 3: The Smart Loop (Years 5+)
Target: Dynamic diseases (Diabetes, Hypertension).
Action: Integrate depots with biosensors for closed-loop modulation.
Goal: The “Artificial Organ.” The device manages the disease autonomously.
Budget & Resource Allocation
To execute this strategy, capital must be aggressively reallocated from “Path A” (Sustaining) to “Path B” (Disruptive).
The “Delete” List (Divestment)
Marketing Agencies: Slash the budget for “Patient Education” and “Adherence Counseling” materials by 80%. You cannot educate away entropy.
Digital Health Apps: Divest from “Reminder Apps” and “Gamification” pilots. These are Band-Aids.
Smart Packaging: Stop funding Bluetooth pill bottles. They optimize a dying form factor.
The “Invest” List (Acquisition)
Materials Science (50% of R&D): Pour capital into hydrogels, bio-erodible polymers (PLGA), and depot formulation technologies. The new IP battlefield is not the molecule, but the matrix that holds it.
MEMS & Micro-Fluidics (30% of R&D): Invest in solid-state micropumps and silicon-based drug reservoirs for active delivery.
Real-World Evidence (20% of R&D): Fund head-to-head trials proving that “Zero-UI” delivery reduces total cost of care (hospitalizations) compared to “Daily Pill” standard of care.
Visuals for the Board Deck
Figure 1: The “Sawtooth” vs. The “Flatline”: A pharmacokinetic graph showing the chaotic peaks and troughs of oral dosing vs. the steady state of an implant.
Figure 2: The ID10T Cost Curve: A comparison of the “Total Cost of Delivery” (Drug + Shadow Labor + Rescue Care) for Pills vs. Implants.
Figure 3: The Hierarchy of Intervention: A pyramid showing the evolution from Manual (Pill) -> Assisted (Smart Bottle) -> Autonomous (Implant).
If you find my writing thought-provoking, please give it a thumbs up and/or share it. If you think I might be interesting to work with, here’s my contact information (my availability is limited):
Book an appointment: https://pjtbd.com/book-mike
Email me: mike@pjtbd.com
Call me: +1 678-824-2789
Join the community: https://pjtbd.com/join
Follow me on 𝕏: https://x.com/mikeboysen
Articles - jtbd.one - De-Risk Your Next Big Idea
New Masterclass: Principle to Priority
Q: Does your innovation advisor provide a 6-figure pre-analysis before delivering the 6-figure proposal?